Medical Affairs Outsourcing Market Growth Opportunities and Forecast till 2032
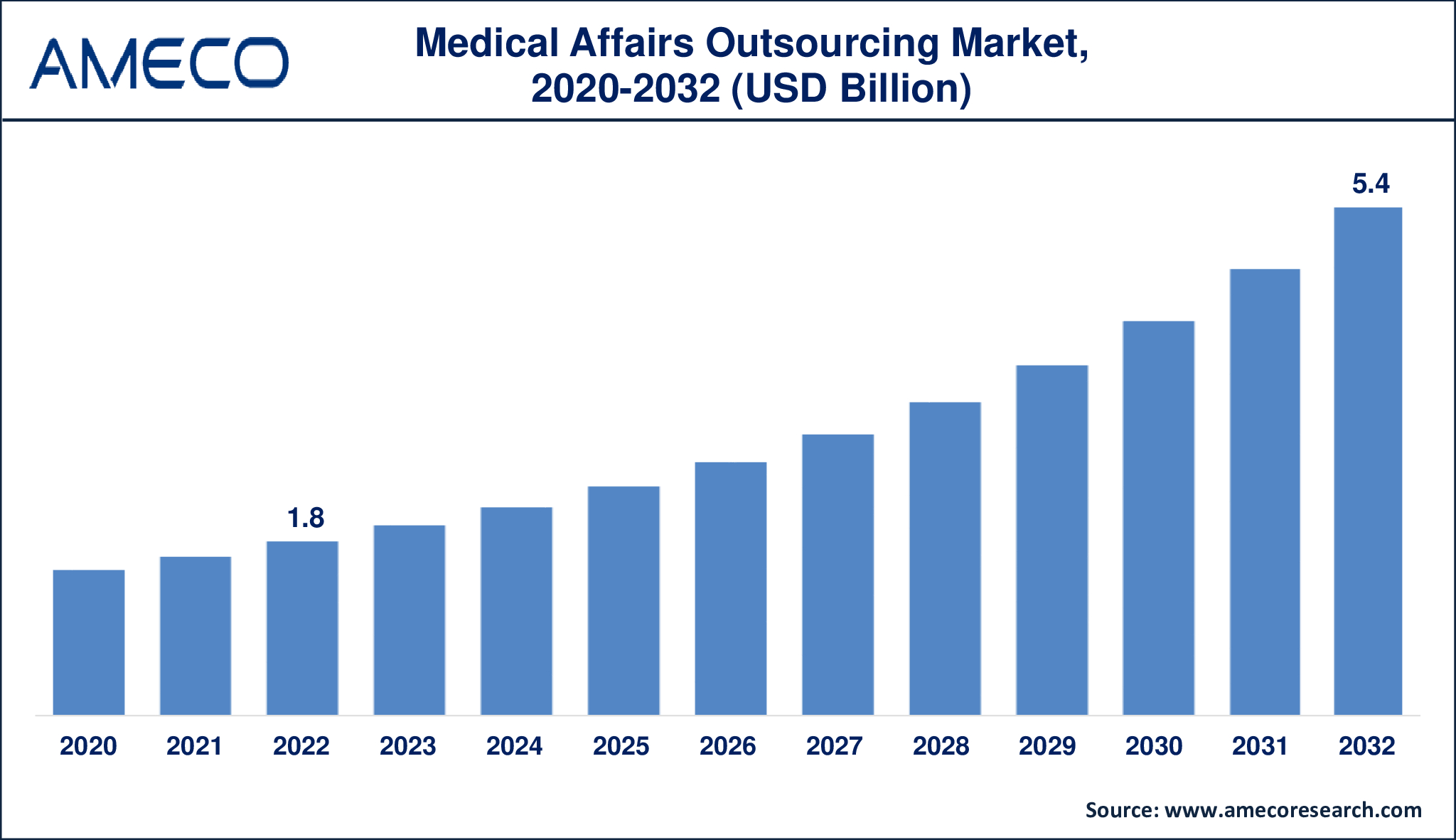
The Global Medical Affairs Outsourcing Market Size was valued at USD 1.8 Billion in 2022 and is anticipated to reach USD 5.4 Billion by 2032 with a CAGR of 11.6% from 2023 to 2032.
Medical affairs outsourcing is the practice in which pharmaceutical, biotechnology, and medical device businesses hire external service providers to execute various medical affairs activities. These responsibilities include medical writing, regulatory filings, pharmacovigilance, medical information management, and clinical trial assistance. Companies that outsource these tasks can benefit from specialized expertise, increase productivity, and lower operational expenses. This method enables internal teams to concentrate on key operations such as R&D, strategic planning, and market expansion. Outsourcing partners often provide in-depth knowledge of regulatory standards, therapeutic areas, and current industry trends, ensuring that the company's medical affairs are managed properly and compliantly.
Medical affairs outsourcing is being driven by the growing complexity of drug development and commercialization processes, severe regulatory constraints, and the need for cost-effective operations. Companies are under increasing pressure to reduce the time it takes to bring new goods to market while maintaining high safety and efficacy levels. Outsourcing allows access to a worldwide talent pool and advanced technical resources that would otherwise be unavailable in-house. Furthermore, it provides scalability, allowing businesses to shift resources based on project demands without the long-term commitment associated with full-time employees. As the pharmaceutical industry evolves, medical affairs outsourcing remains a strategic tool for organizations looking to gain a competitive advantage while also ensuring regulatory compliance.
|
Parameter |
Medical Affairs Outsourcing Market |
|
Medical Affairs Outsourcing Market Size in 2022 |
US$ 1.8 Billion |
|
Medical Affairs Outsourcing Market Forecast By 2032 |
US$ 5.4 Billion |
|
Medical Affairs Outsourcing Market CAGR During 2023 – 2032 |
11.6% |
|
Medical Affairs Outsourcing Market Analysis Period |
2020 - 2032 |
|
Medical Affairs Outsourcing Market Base Year |
2022 |
|
Medical Affairs Outsourcing Market Forecast Data |
2023 - 2032 |
|
Segments Covered |
By Services, By Industry, and By Region |
|
Medical Affairs Outsourcing Market Regional Scope |
North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
|
Key Companies Profiled |
Ashfield Healthcare Communications, ICON plc, Indegene Inc., IQVIA Holdings Inc, Pharmaceutical Product Development, LLC, SGS SA, Syneos Health Inc, The Medical Affairs Company (TMAC), Wuxi Clinical Development, Inc., and ZEINCRO Group. |
|
Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Regulation Analysis |
Medical Affairs Outsourcing Market Dynamics
The medical affairs outsourcing industry is defined by a dynamic interaction of many elements that drive its expansion. One of the key reasons is the growing complexity and amount of regulatory requirements that pharmaceutical, biotechnology, and medical device businesses must meet. As regulatory authorities throughout the world tighten their requirements and establish new rules, there is a growing demand for specialist expertise in regulatory submissions, compliance, and pharmacovigilance. Outsourcing these operations to professional service providers enables businesses to assure regulatory compliance while freeing up internal resources for essential research and development activities. Furthermore, the demand for comprehensive and compliance medical writing, data management, and clinical trial support has fueled the outsourcing trend.
Cost-effectiveness and operational flexibility are also important factors driving the growth of the medical affairs outsourcing industry. In today's highly competitive sector, businesses are constantly under pressure to cut costs and enhance operational efficiency. Outsourcing is a viable alternative because it provides access to a worldwide pool of specialized personnel and advanced technology resources without the long-term financial commitment required to sustain large in-house teams. This method not only helps to reduce operational costs, but it also gives you the flexibility to scale resources up or down dependent on project requirements. Furthermore, outsourcing partners frequently introduce best practices and novel solutions, which improve the overall efficiency and quality of medical affairs tasks.
Market dynamics are also influenced by companies' strategic need to focus on their core capabilities and shorten time-to-market for new products. In a time-sensitive sector, outsourcing non-core but crucial tasks like medical information administration and clinical trial support can drastically accelerate product development and approval processes. Furthermore, the advent of customized care and the growing emphasis on patient-centered approaches have necessitated more specialized and complex medical affairs operations, which are frequently better handled by outside professionals. As the drug research and commercialization landscape evolves, the medical affairs outsourcing market is likely to expand, driven by the demand for specialized knowledge, cost-effective operations, and a strategic emphasis on innovation and core activities.
Global Medical Affairs Outsourcing Market Segment Analysis
Medical Affairs Outsourcing Market By Services
· Medical Monitoring
· Medical Writing & Publishing
· Medical Information
· Medical Science Liaisons
· Others
Medical writing and publishing services have dominated the medical affairs outsourcing business, owing to the growing complexity and number of regulatory documents required for drug approval procedures. These services include the creation of clinical research reports, regulatory submissions, safety updates, and scientific publications, all of which are required for compliance and successful market entrance. The demand for high-quality, compliance medical writing has increased as businesses attempt to meet tight regulatory requirements and effectively communicate their research findings. This dominance is strengthened by the demand for specialized expertise and painstaking attention to detail, both of which outsourcing companies excel at providing.
Medical Affairs Outsourcing Market By Industry
· Medical Devices
· Pharmaceutical
· Biopharmaceutical
The pharmaceutical industry has led the Medical Affairs Outsourcing Market, owing to the high demand for regulatory compliance, clinical trial support, and effective transmission of complicated scientific information. Pharmaceutical firms are subjected to stringent regulatory scrutiny and must provide a wide range of paperwork, including clinical trial results, regulatory submissions, and post-market surveillance. The complexities of drug development, combined with the need to follow high regulatory standards around the world, have prompted major corporations to seek out specialized outsourcing partners. This trend is exacerbated by the need to reduce time-to-market for new treatments, manage costs, and use sophisticated expertise, making outsourcing a critical strategy in the pharmaceutical industry.
Medical Affairs Outsourcing Market Regional Analysis
The Medical Affairs Outsourcing Market demonstrates various regional patterns caused by differences in regulatory environments, healthcare infrastructure, and the presence of important industry players. North America, notably the United States, dominates the market due to its powerful pharmaceutical and biopharmaceutical sectors, combined with severe regulatory requirements imposed by the FDA. The strong demand for regulatory compliance, enhanced clinical trial support, and specialist medical writing services drives the outsourcing market in this region. Furthermore, the presence of a large number of outsourcing service providers with excellent technological skills and significant industry knowledge strengthens North America's market position.
Europe follows closely, with major contributions from Germany, the United Kingdom, and France. The European market is propelled by the region's tight regulatory structure and the growing complexity of clinical trials and drug clearance processes controlled by authorities such as the European Medicines Agency (EMA). The region's emphasis on cost-efficiency and the necessity to simplify operations in a competitive environment have fueled demand for outsourcing services even more. In contrast, Asia-Pacific is emerging as the fastest-growing area in the Medical Affairs Outsourcing Market. This expansion is due to increased clinical trial activity, positive regulatory developments, and cost advantages provided by nations such as China, India, and Japan. The region's expanding pharmaceutical and biopharmaceutical sectors, combined with a growing emphasis on innovative healthcare solutions, place Asia-Pacific as a key player in the future growth of the medical affairs outsourcing industry.
Medical Affairs Outsourcing Market Leading Companies
The medical affairs outsourcing market players profiled in the report are Ashfield Healthcare Communications, ICON plc, Indegene Inc., IQVIA Holdings Inc, Pharmaceutical Product Development, LLC, SGS SA, Syneos Health Inc, The Medical Affairs Company (TMAC), Wuxi Clinical Development, Inc., and ZEINCRO Group.
Medical Affairs Outsourcing Market Regions
North America
· U.S.
· Canada,
Europe
· U.K.
· Germany
· France
· Spain
· Rest of Europe
Latin America
· Brazil
· Mexico
· Rest of Latin America
Asia-Pacific
· China
· Japan
· India
· Australia
· South Korea
· Rest of Asia-Pacific
Middle East & Africa
· GCC
· South Africa
· Rest of Middle East & Africa